FDA to re-evaluate effectiveness of common nasal congestion ingredient

A free daily digest of the biggest news stories of the day - and the best features from our website
Thank you for signing up to TheWeek. You will receive a verification email shortly.
There was a problem. Please refresh the page and try again.
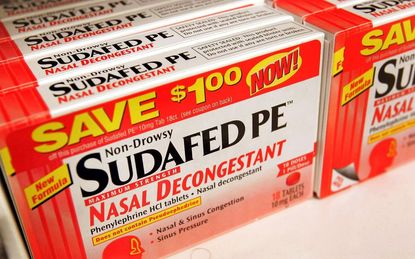
The Food and Drug Administration (FDA) will convene a panel next week to re-evaluate the effectiveness of oral phenylephrine, a common ingredient found in numerous over-the-counter decongestants. The panel is slated meet Monday and Tuesday, just days after the FDA released a report claiming that phenylephrine likely doesn't work.
The panel will examine the potential benefits and drawbacks of phenylephrine and question medical advisers. The drug, which was first approved by the FDA in the 1970s, can be found in many varieties of anti-cold medications, including versions of Nyquil, Sudafed, Benadryl, Vicks and Mucinex.
These medications are currently classified as being "generally recognized as safe and effective," according to an FDA fact sheet. However, the FDA's recent report, released last Thursday, claimed that phenylephrine is unlikely to work in any dosages, putting that classification at high risk of being revoked.
Subscribe to The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
"Because this would represent a major change in the agency's position, we believe that presenting this information in an open public forum, along with a full discussion and vote" from committee members, "will be extremely helpful," the FDA's report said.
If phenylephrine does end up being reclassified, major drug companies like Kenvue, Procter & Gamble, Reckitt Benckiser and others would likely have to reformulate many of their flagship decongestants or pull them from store shelves, Bloomberg reported.
The effectiveness of phenylephrine has long been debated. A 2015 clinical trial of 500 adults with seasonal allergies found that phenylephrine was "not significantly better than placebo at relieving nasal congestion in adults." Dr. Wynne Armand, a primary care doctor at Massachusetts General Hospital, told NBC News she advises patients with cold symptoms "to avoid buying oral meds that have phenylephrine," and won't prescribe it.

Continue reading for free
We hope you're enjoying The Week's refreshingly open-minded journalism.
Subscribed to The Week? Register your account with the same email as your subscription.
Sign up to our 10 Things You Need to Know Today newsletter
A free daily digest of the biggest news stories of the day - and the best features from our website
Justin Klawans is a staff writer at The Week. Based in Chicago, he was previously a breaking news reporter for Newsweek, writing breaking news and features for verticals including politics, U.S. and global affairs, business, crime, sports, and more. His reporting has been cited on many online platforms, in addition to CBS' The Late Show with Stephen Colbert.
He is also passionate about entertainment and sports news, and has covered film, television, and casting news as a freelancer for outlets like Collider and United Press International, as well as Chicago sports news for Fansided.
-
 Ben Fountain's 6 favorite books about Haiti
Ben Fountain's 6 favorite books about HaitiFeature The award-winning author recommends works by Marie Vieux-Chauvet, Katherine Dunham and more
By The Week Staff Published
-
 6 picturesque homes in apartments abroad
6 picturesque homes in apartments abroadFeature Featuring a wall of windows in Costa Rica and a luxury department store-turned-home in New Zealand
By The Week Staff Published
-
 Why 2023 has been the year of strikes and labor movements
Why 2023 has been the year of strikes and labor movementsThe Explainer From Hollywood to auto factories, workers are taking to the picket lines
By Justin Klawans Published
-
 Popular weight loss drugs linked to higher risk of serious gastrointestinal problems
Popular weight loss drugs linked to higher risk of serious gastrointestinal problemsSpeed Reads Researchers found that semaglutide, the active ingredient in Wegovy and Ozempic, had a higher risk for side effects like stomach paralysis.
By Theara Coleman Published
-
 What is a 'feminist approach' to cancer care?
What is a 'feminist approach' to cancer care?The Explainer 800,000 women die from 'preventable' cancers each year due to 'patriarchy', landmark study finds
By Harriet Marsden, The Week UK Published
-
 Animals and plants that have been used to fight disease
Animals and plants that have been used to fight diseaseThe Explainer The world's flora and fauna have long been medically important
By Devika Rao Published
-
 Heat harms the brain more than we think
Heat harms the brain more than we thinkWarmer temperatures could be affecting us mentally
By Devika Rao Published
-
 A flesh-eating bacteria is growing in numbers due to climate change
A flesh-eating bacteria is growing in numbers due to climate changeSpeed Read
By Devika Rao Published
-
 CDC recommends new RSV vaccine for infants under 8 months
CDC recommends new RSV vaccine for infants under 8 monthsSpeed Read
By Devika Rao Published
-
 Will Medicare drug price controls save lives?
Will Medicare drug price controls save lives?Talking Point Medicare starts negotiating lower drug prices over Big Pharma protests
By Harold Maass Published
-
 U.S. health agency advises easing federal marijuana restrictions
U.S. health agency advises easing federal marijuana restrictionsSpeed Read
By Peter Weber Published